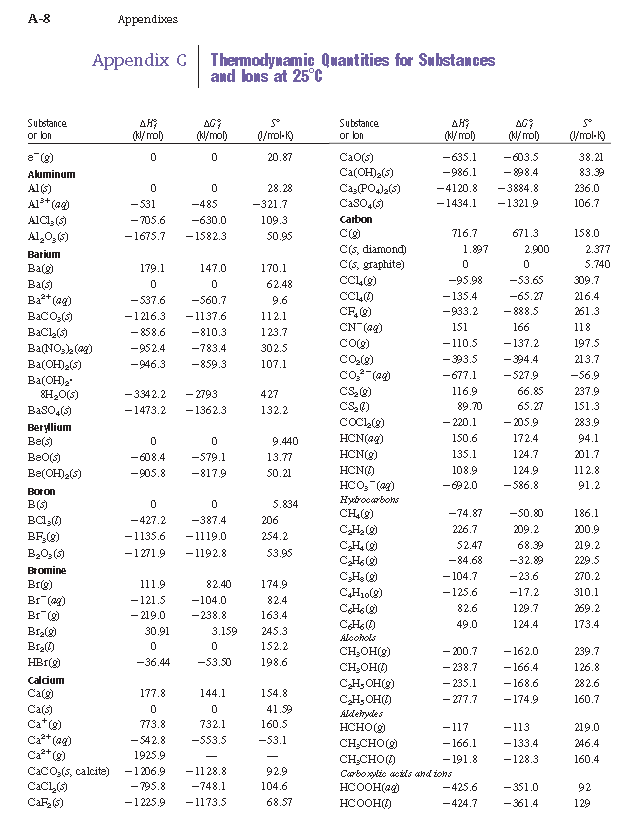
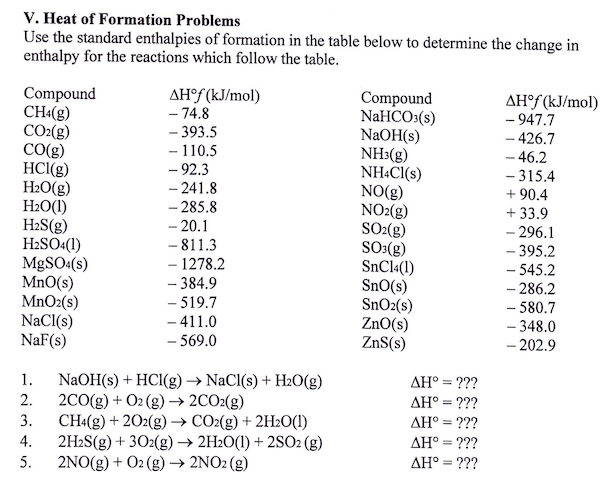
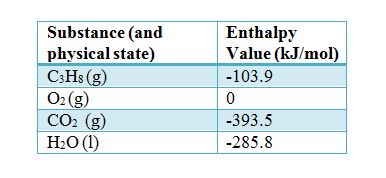
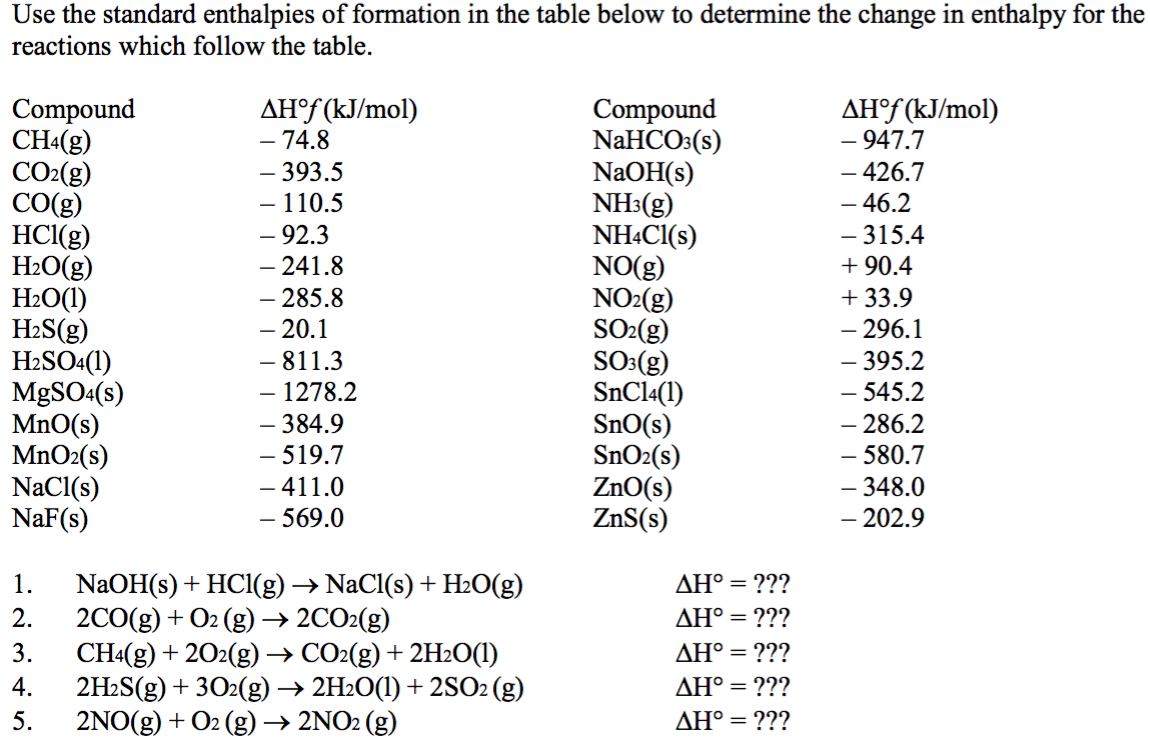
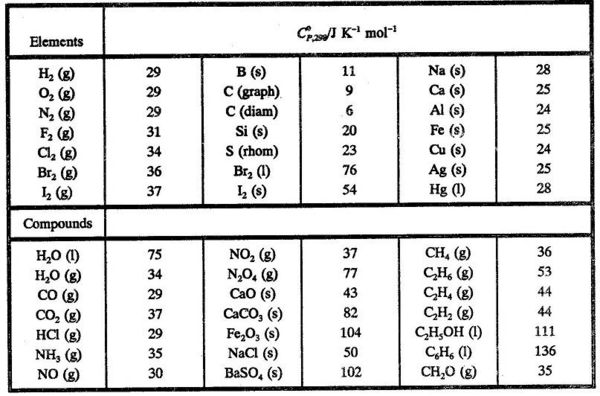
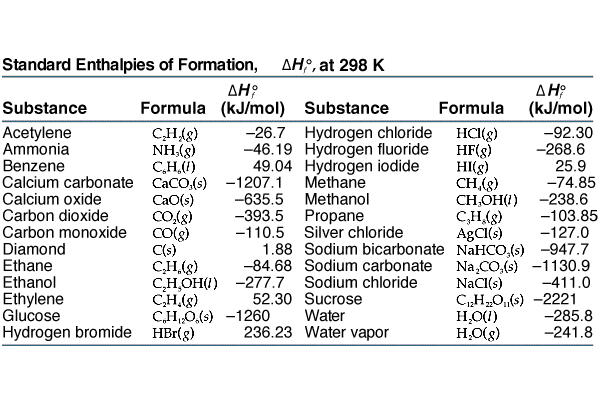
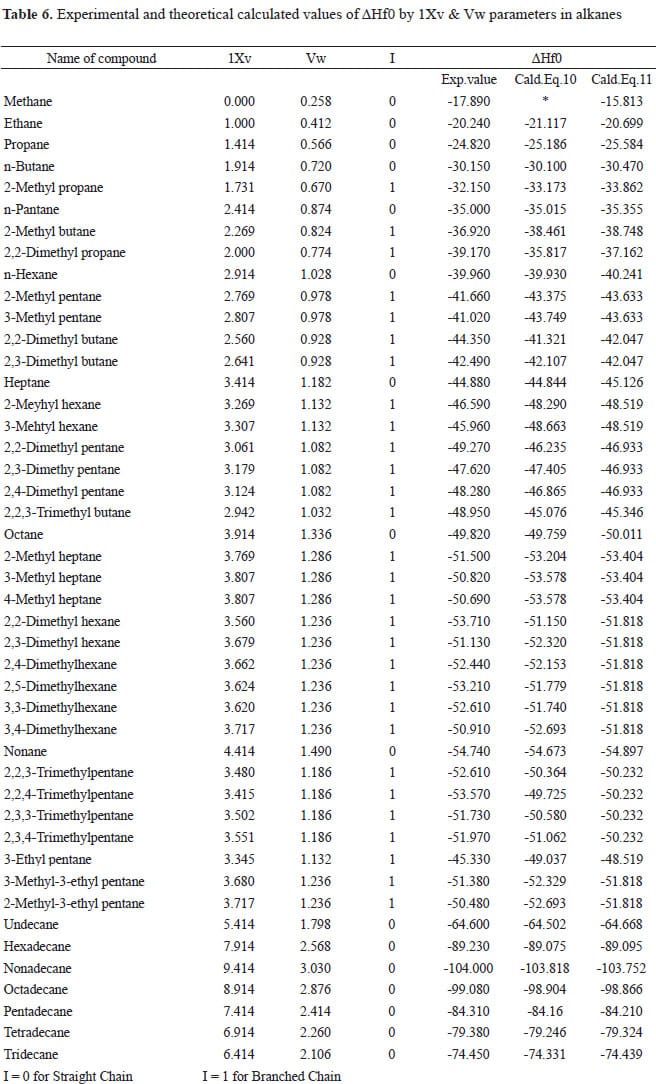
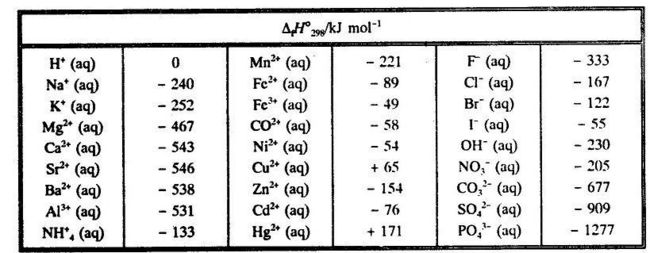
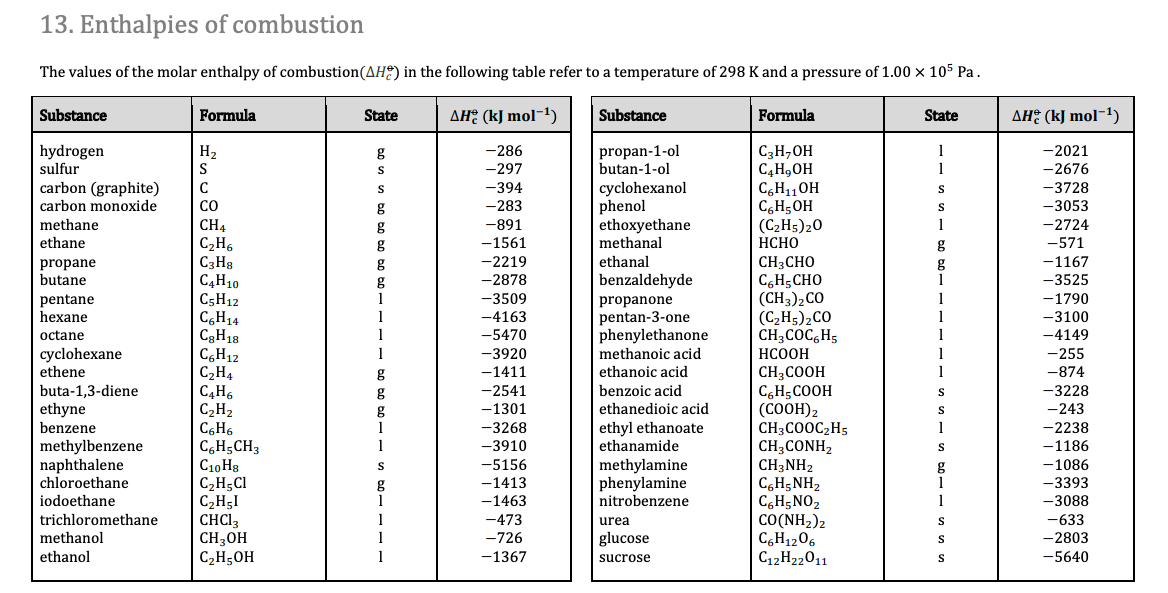
density functional theory - How to calculate the enthalpy of formation? - Matter Modeling Stack Exchange
Calculate the enthalpy of reaction for the reaction "CH"_3"COOH" + "H"_2"O" -> "CH"_3"CH"_2"OH" + "O"_2? | Socratic

90 POINTSSSS!!! Step 3: Determine the amount of energy change in the reaction. a) Use the table of enthalpy - brainly.com
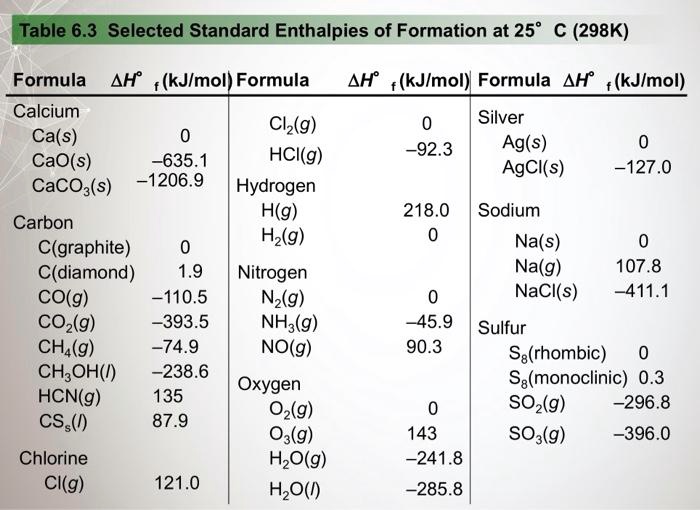
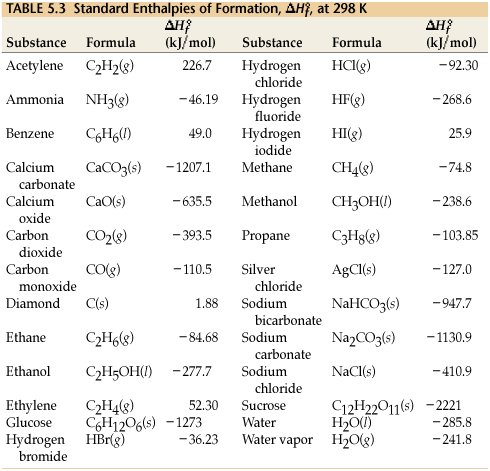
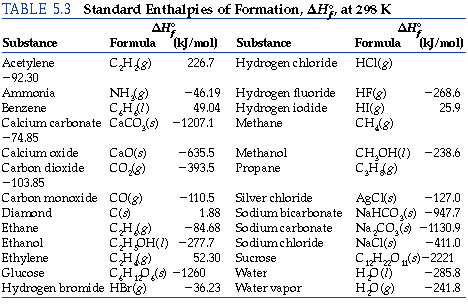
SOLVED: Table 6.3 Selected Standard Enthalpies of Formation at 25°C (298K) Formula ΔH° (kJ/mol) Formula ΔH° (kJ/mol) Formula ΔH° f (kJ/mol) Calcium Cl2(g) -92.3 Ag(s) 0 CaO(s) -635.0 HCI(g) -127.0 AgCl(s) -92.3

Table 3 from Group additivity values for enthalpies of formation (298 K), entropies (298 K), and molar heat capacities (300 K < T < 1500 K) of gaseous fluorocarbons | Semantic Scholar
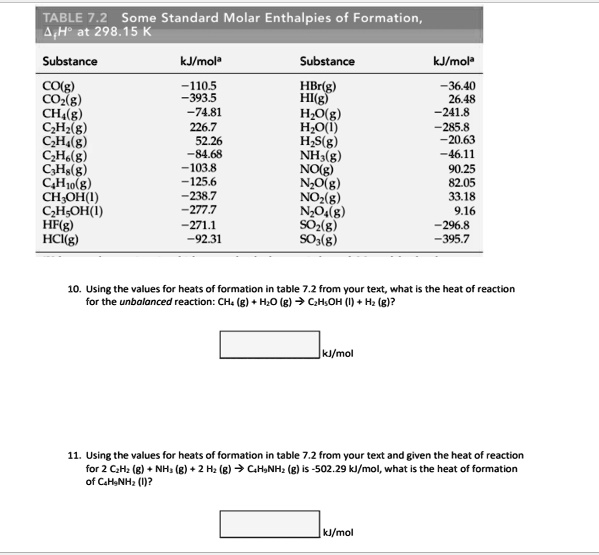
SOLVED: TABLE 7.2 Some Standard Molar Enthalpies of Formation; Heat at 298.15 K Substance kJ/mol Substance kJ/mol CO(g) CO2(g) CH4(g) C2H6(g) C3H8(g) C4H10(g) C6H6(g) C6H12(g) C6H14(g) C6H6OH(l) C2H5OH(l) -105 -393.5 -74.81 226.7

Table VII from Large-scale calculations of gas phase thermochemistry: Enthalpy of formation, standard entropy, and heat capacity | Semantic Scholar
![Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book] Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book]](https://www.oreilly.com/api/v2/epubs/9780132885478/files/graphics/appd-tab-d1a.jpg)